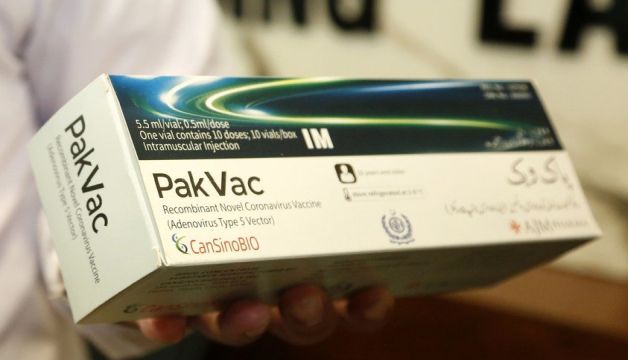
PakVac, the first locally packaged single-dose COVID-19 vaccine in Pakistan, is acceptable in the US and UK, SAPM on Health said, said Dr. Faisal Sultan.
During a talk show, Dr. Faisal Sultan made it clear that PakVac is acceptable in all countries, including the US and the UK, where arriving travelers do not need to get vaccinated against coronavirus with certain vaccines.
PakVac, also known as Ad5-nCoV, is a prestigious version of a Chinese single-dose coronavirus vaccine originally developed by CanSinoBIO in association with the Beijing Institute of Biotechnology. The vaccine has been tested in large-scale phase III clinical trials in five countries, including Pakistan, involving more than 40,000 volunteers.
Also Read: Pakistan to Start The Manufacturing of its Own Covid-19 Vaccine Soon
The results of the studies were published in February this year and confirmed that the CanSinoBIO vaccine was 65% effective in preventing symptomatic cases and 91% in preventing serious infections.
For the Pakistan subgroup, the vaccine provided 75% protection against symptomatic cases and 100% against serious infections.
In May, the National Institute of Health (NIH) in Islamabad launched PakVac’s local packaging after its raw material arrived in Pakistan from China.
The NIH manufactures 3 million doses of PakVac each month, greatly reducing Pakistan’s reliance on imported COVID-19 vaccines and ensuring adequate availability of the coronavirus vaccine.
Also Read: US Govt Announces to Send 2.5 Mln Doses of Moderna Covid-19 Vaccine to Pakistan Next Week